Prof. Dr. Martin Muhler
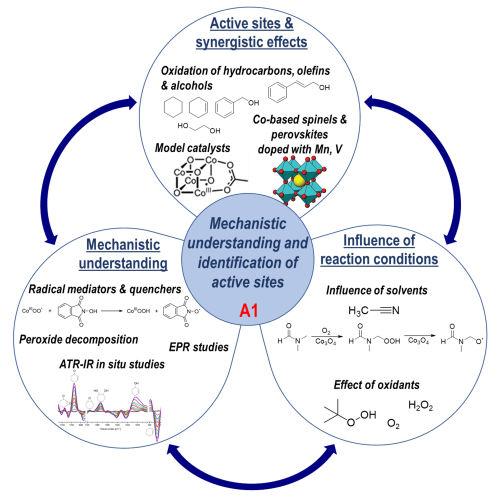
Various transition metal oxide catalysts are applied in different oxidation reactions to comprehensively assess their chemical reactivity by finding activity and selectivity patterns in a reaction matrix. The reaction conditions are chosen at temperatures below 200 °C in the liquid phase to guarantee conservation of the kinetic control of specific materials properties such as defects that have been deliberately adjusted by targeted catalyst synthesis. The catalysts will be cobalt-based spinel and perovskite oxides including Fe, Mn, or V substitution obtained from the projects of area C and characterized thoroughly by the projects of area B. The reaction matrix is formed by variation of the substrate using aromatic alcohols like cinnamyl alcohol, diols like ethylene glycol, cyclic alkenes like cyclohexene, alkanes like cyclohexane, the oxidant using O2 and peroxides such as H2O2 and tert-butyl hydroperoxide (TBHP), and the solvent comprising water, acetonitrile, and dimethylformamide.
Within the first funding period, the CRC’s Comparative Study delivered generic reactivity trends that allow the establishment and validation of structure–activity correlations. In the second funding period, this systematic approach will be extended to obtain mechanistic understanding and identification of active sites in close collaboration with the electrochemical projects. Because hydroperoxides are the key intermediates in the oxidation of cyclohexene and cyclohexane, separate decomposition experiments will be performed. A combination of radical initiator and radical scavenger experiments and EPR investigations will be routinely conducted to elucidate the radical mechanisms. In addition, molecular model catalysts will be also tested, which mimic domains of the real structure of the catalysts and help to identify the active sites. Finally, a continuously operated three-phase capillary reactor will be established, which is especially suitable to study long-term stability, and a heated high-pressure batch reactor equipped with electrodes will be built jointly, enabling both thermal and electrochemical kinetic studies in close collaboration with the electrochemical projects.
(Figure: Schematic representation of the applied methods for mechanistic understanding).